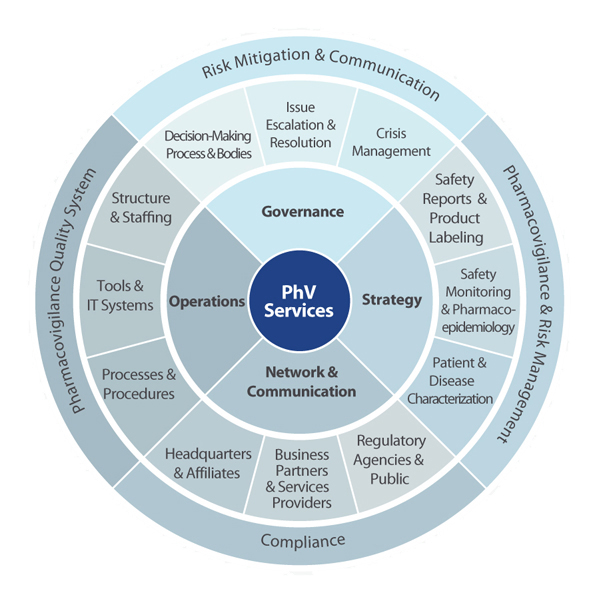
CPVS provides a broad spectrum of drug safety services tailored to the needs and requirements of our clients. Our experts have many years of experience developing innovative and highly-customizable solutions for the drug safety management of both investigational drugs and marketed products. We help manage the safety of the client’s products with a regulatory-compliant and best-practices approach.
European Qualified Person at Pharmacovigilance (QPPV)
• Establishment and maintenance of pharmacovigilance systems.
• European Economic Area (EEA) Qualified Person for Pharmacovigilance (& deputy QPPV).
Local Pharmacovigilance Representative
• Local pharmacovigilance representative (eg, in Spain).
• Direct interaction with local regulators on pharmacovigilance and regulatory matters.
Pharmacovigilance System
• Definition of pharmacovigilance and quality systems.
• Services include: Creation of PSMF, DDPS to PSMF transition, Managing PSMF updates and Template and SOP development
Case Management Solutions
• Analysing, assessing and reporting important safety data requires best practices for data entry, follow-up and query, quality control, medical review and quality assurance. From clinical trials safety to post-marketing surveillance, CPVS manages data collection, triage, tracking, and reporting of your adverse events from start to finish.
• Elaboration and testing of business continuity plans (eg, information technology system failure; disaster recovery; contingencies).
Medical Information Support
• CPVS can provide highest quality medical information service to healthcare professionals and patients by ensuring all enquiries receive accurate and medically sound responses, whilst meeting regulatory and legal requirements.
Pharmacovigilance Agreements with Third Parties
• Generation and maintenance of pharmacovigilance agreements.
• Establishment of clear communication channels and methods with partners.
Aggregate Reports Solutions
• Our group consists of a professional team of physicians and regulatory experts that work with you to assist with the continuous cycle of preparing global aggregate regulatory reporting.
• Our expertise in both pre and post-marketing pharmacovigilance regulations, global marketing authorisation applications, variations and renewals. CPVS is able to lead all global regulatory report generation and submission services designed to align with electronic or paper submissions.
• Some examples of reports delivered:
- Global periodic safety reporting (PADER, PSUR/PBRER)
- Provide support for ASR/DSUR
- NDA and IND submissions (supporting preparation of IND/NDA annual reports, NDA ISS sections and clinical study reports)
- Regulatory report submissions to appropriate authorities
- Medication error and drug abuse/misuse reporting
- Toxicology report management
- Medical writing support for report generation
- Other special reports required by regulatory authorities (e.g. risk management associated reports and other post-marketing commitments
Signal Surveillance Activities
CPVS signal detection group consist of highly experienced safety physicians, safety scientists, and medical writers. The group is responsible for lifecycle signal detection and analysis as well as recommendations and proposals to clients regarding findings and additional analysis.
Risk Management System
• Development of RMP/REMS.
• Pharmacovigilance plans
• Assistance in defining pharmacovigilance actions, risk minimisation and risk prevention strategies.
• Reference safety information reviews/revisions (e.g. core label, SPC, PI, CIB)
• Medical monitoring
• Risk-Benefit Evaluation / Epidemiology
Literature Search Solutions
• CPVS can conduct weekly, monthly and quarterly internationally qualified scientific review of adverse events from multiple databases to ensure that you have complete and diligent product case management and reporting.
• We conduct periodic search and review on articles, manuscripts, abstracts, excerpts, bibliographic searches for Individual Case Safety Reports (ICSR), PSURs, annual reports, risk management analysis, and other ad hoc safety reports.
Medical Writing Services
CPVS offers a range of medical writing services to support your operations. Our experienced team is well-versed in preparing a variety of documents necessary for safety reporting, risk management and regulatory compliance.